- Overview
-
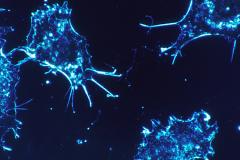
Most chemotherapeutic agents used to treat childhood malignant tumours target the cellular machinery involved in replicating the DNA of rapidly dividing cells. However, these agents do not distinguish malignant from normal proliferating cells, and therefore may damage the growing child's development. Increasing our understanding of how tumour cells respond to the signals, from outside or within the cell, that tell it to divide or not will give us insights into the pathways specific to tumour cells. Studying these pathways, collectively known as cellular signal transduction pathways, is central to understanding tumour cell biology. Targeting these pathways can be used as a treatment strategy, while minimizing effects on normal growth. The focus of my research group is the investigation of how gene rearrangements or mutations disrupt signal transduction in pediatric malignant tumours, or how genome-wide changes in gene expression relate to signalling alterations in these tumours. The ultimate goal is to identify new therapeutic targets in childhood cancer.
- Publications
-
Supplementary Fig. S5 from Phase Ib Pharmacodynamic Study of the MNK Inhibitor Tomivosertib (eFT508) Combined With Paclitaxel in Patients With Refractory Metastatic Breast Cancer
Cristiano Ferrario and John Mackey and Karen A. Gelmon and Nathalie Levasseur and Poul H. Sorensen and Htoo Zarni Oo and Gian L. Negri and Veronica W.L. Tse and Sandra E. Spencer and Grace Cheng and Gregg B. Morin and Sonia del Rincon and Tiziana Cotechini and Christophe Gonçalves and Charles C.T. Hindmarch and Wilson H. Miller and Mehdi Amiri and Tayebeh Basiri and Victor Villareal-Corpuz and Sam Sperry and Kevin Gregorczyk and Gonzalo Spera and Nahum Sonenberg and Michael Pollak
DOI: 10.1158/1078-0432.28332736
02/2025Supplementary Table S9 from Phase Ib Pharmacodynamic Study of the MNK Inhibitor Tomivosertib (eFT508) Combined With Paclitaxel in Patients With Refractory Metastatic Breast Cancer
Cristiano Ferrario and John Mackey and Karen A. Gelmon and Nathalie Levasseur and Poul H. Sorensen and Htoo Zarni Oo and Gian L. Negri and Veronica W.L. Tse and Sandra E. Spencer and Grace Cheng and Gregg B. Morin and Sonia del Rincon and Tiziana Cotechini and Christophe Gonçalves and Charles C.T. Hindmarch and Wilson H. Miller and Mehdi Amiri and Tayebeh Basiri and Victor Villareal-Corpuz and Sam Sperry and Kevin Gregorczyk and Gonzalo Spera and Nahum Sonenberg and Michael Pollak
DOI: 10.1158/1078-0432.28332691
02/2025Supplementary Table S5 from Phase Ib Pharmacodynamic Study of the MNK Inhibitor Tomivosertib (eFT508) Combined With Paclitaxel in Patients With Refractory Metastatic Breast Cancer
Cristiano Ferrario and John Mackey and Karen A. Gelmon and Nathalie Levasseur and Poul H. Sorensen and Htoo Zarni Oo and Gian L. Negri and Veronica W.L. Tse and Sandra E. Spencer and Grace Cheng and Gregg B. Morin and Sonia del Rincon and Tiziana Cotechini and Christophe Gonçalves and Charles C.T. Hindmarch and Wilson H. Miller and Mehdi Amiri and Tayebeh Basiri and Victor Villareal-Corpuz and Sam Sperry and Kevin Gregorczyk and Gonzalo Spera and Nahum Sonenberg and Michael Pollak
DOI: 10.1158/1078-0432.28332709
02/2025Supplementary Fig. S2 from Phase Ib Pharmacodynamic Study of the MNK Inhibitor Tomivosertib (eFT508) Combined With Paclitaxel in Patients With Refractory Metastatic Breast Cancer
Cristiano Ferrario and John Mackey and Karen A. Gelmon and Nathalie Levasseur and Poul H. Sorensen and Htoo Zarni Oo and Gian L. Negri and Veronica W.L. Tse and Sandra E. Spencer and Grace Cheng and Gregg B. Morin and Sonia del Rincon and Tiziana Cotechini and Christophe Gonçalves and Charles C.T. Hindmarch and Wilson H. Miller and Mehdi Amiri and Tayebeh Basiri and Victor Villareal-Corpuz and Sam Sperry and Kevin Gregorczyk and Gonzalo Spera and Nahum Sonenberg and Michael Pollak
DOI: 10.1158/1078-0432.28332745
02/2025Supplementary Table S6 from Phase Ib Pharmacodynamic Study of the MNK Inhibitor Tomivosertib (eFT508) Combined With Paclitaxel in Patients With Refractory Metastatic Breast Cancer
Cristiano Ferrario and John Mackey and Karen A. Gelmon and Nathalie Levasseur and Poul H. Sorensen and Htoo Zarni Oo and Gian L. Negri and Veronica W.L. Tse and Sandra E. Spencer and Grace Cheng and Gregg B. Morin and Sonia del Rincon and Tiziana Cotechini and Christophe Gonçalves and Charles C.T. Hindmarch and Wilson H. Miller and Mehdi Amiri and Tayebeh Basiri and Victor Villareal-Corpuz and Sam Sperry and Kevin Gregorczyk and Gonzalo Spera and Nahum Sonenberg and Michael Pollak
DOI: 10.1158/1078-0432.28332703
02/2025Supplementary Table S8 from Phase Ib Pharmacodynamic Study of the MNK Inhibitor Tomivosertib (eFT508) Combined With Paclitaxel in Patients With Refractory Metastatic Breast Cancer
Cristiano Ferrario and John Mackey and Karen A. Gelmon and Nathalie Levasseur and Poul H. Sorensen and Htoo Zarni Oo and Gian L. Negri and Veronica W.L. Tse and Sandra E. Spencer and Grace Cheng and Gregg B. Morin and Sonia del Rincon and Tiziana Cotechini and Christophe Gonçalves and Charles C.T. Hindmarch and Wilson H. Miller and Mehdi Amiri and Tayebeh Basiri and Victor Villareal-Corpuz and Sam Sperry and Kevin Gregorczyk and Gonzalo Spera and Nahum Sonenberg and Michael Pollak
DOI: 10.1158/1078-0432.28332697
02/2025Supplementary Fig. S4 from Phase Ib Pharmacodynamic Study of the MNK Inhibitor Tomivosertib (eFT508) Combined With Paclitaxel in Patients With Refractory Metastatic Breast Cancer
Cristiano Ferrario and John Mackey and Karen A. Gelmon and Nathalie Levasseur and Poul H. Sorensen and Htoo Zarni Oo and Gian L. Negri and Veronica W.L. Tse and Sandra E. Spencer and Grace Cheng and Gregg B. Morin and Sonia del Rincon and Tiziana Cotechini and Christophe Gonçalves and Charles C.T. Hindmarch and Wilson H. Miller and Mehdi Amiri and Tayebeh Basiri and Victor Villareal-Corpuz and Sam Sperry and Kevin Gregorczyk and Gonzalo Spera and Nahum Sonenberg and Michael Pollak
DOI: 10.1158/1078-0432.28332739
02/2025Supplementary Fig. S3 from Phase Ib Pharmacodynamic Study of the MNK Inhibitor Tomivosertib (eFT508) Combined With Paclitaxel in Patients With Refractory Metastatic Breast Cancer
Cristiano Ferrario and John Mackey and Karen A. Gelmon and Nathalie Levasseur and Poul H. Sorensen and Htoo Zarni Oo and Gian L. Negri and Veronica W.L. Tse and Sandra E. Spencer and Grace Cheng and Gregg B. Morin and Sonia del Rincon and Tiziana Cotechini and Christophe Gonçalves and Charles C.T. Hindmarch and Wilson H. Miller and Mehdi Amiri and Tayebeh Basiri and Victor Villareal-Corpuz and Sam Sperry and Kevin Gregorczyk and Gonzalo Spera and Nahum Sonenberg and Michael Pollak
DOI: 10.1158/1078-0432.28332742
02/2025Supplementary Fig. S1 from Phase Ib Pharmacodynamic Study of the MNK Inhibitor Tomivosertib (eFT508) Combined With Paclitaxel in Patients With Refractory Metastatic Breast Cancer
Cristiano Ferrario and John Mackey and Karen A. Gelmon and Nathalie Levasseur and Poul H. Sorensen and Htoo Zarni Oo and Gian L. Negri and Veronica W.L. Tse and Sandra E. Spencer and Grace Cheng and Gregg B. Morin and Sonia del Rincon and Tiziana Cotechini and Christophe Gonçalves and Charles C.T. Hindmarch and Wilson H. Miller and Mehdi Amiri and Tayebeh Basiri and Victor Villareal-Corpuz and Sam Sperry and Kevin Gregorczyk and Gonzalo Spera and Nahum Sonenberg and Michael Pollak
DOI: 10.1158/1078-0432.28332748
02/2025Supplementary Table S2 from Phase Ib Pharmacodynamic Study of the MNK Inhibitor Tomivosertib (eFT508) Combined With Paclitaxel in Patients With Refractory Metastatic Breast Cancer
Cristiano Ferrario and John Mackey and Karen A. Gelmon and Nathalie Levasseur and Poul H. Sorensen and Htoo Zarni Oo and Gian L. Negri and Veronica W.L. Tse and Sandra E. Spencer and Grace Cheng and Gregg B. Morin and Sonia del Rincon and Tiziana Cotechini and Christophe Gonçalves and Charles C.T. Hindmarch and Wilson H. Miller and Mehdi Amiri and Tayebeh Basiri and Victor Villareal-Corpuz and Sam Sperry and Kevin Gregorczyk and Gonzalo Spera and Nahum Sonenberg and Michael Pollak
DOI: 10.1158/1078-0432.28332718
02/2025Supplementary Table S1 from Phase Ib Pharmacodynamic Study of the MNK Inhibitor Tomivosertib (eFT508) Combined With Paclitaxel in Patients With Refractory Metastatic Breast Cancer
Cristiano Ferrario and John Mackey and Karen A. Gelmon and Nathalie Levasseur and Poul H. Sorensen and Htoo Zarni Oo and Gian L. Negri and Veronica W.L. Tse and Sandra E. Spencer and Grace Cheng and Gregg B. Morin and Sonia del Rincon and Tiziana Cotechini and Christophe Gonçalves and Charles C.T. Hindmarch and Wilson H. Miller and Mehdi Amiri and Tayebeh Basiri and Victor Villareal-Corpuz and Sam Sperry and Kevin Gregorczyk and Gonzalo Spera and Nahum Sonenberg and Michael Pollak
DOI: 10.1158/1078-0432.28332721
02/2025Supplementary Fig. S7 from Phase Ib Pharmacodynamic Study of the MNK Inhibitor Tomivosertib (eFT508) Combined With Paclitaxel in Patients With Refractory Metastatic Breast Cancer
Cristiano Ferrario and John Mackey and Karen A. Gelmon and Nathalie Levasseur and Poul H. Sorensen and Htoo Zarni Oo and Gian L. Negri and Veronica W.L. Tse and Sandra E. Spencer and Grace Cheng and Gregg B. Morin and Sonia del Rincon and Tiziana Cotechini and Christophe Gonçalves and Charles C.T. Hindmarch and Wilson H. Miller and Mehdi Amiri and Tayebeh Basiri and Victor Villareal-Corpuz and Sam Sperry and Kevin Gregorczyk and Gonzalo Spera and Nahum Sonenberg and Michael Pollak
DOI: 10.1158/1078-0432.28332730
02/2025Supplementary Fig. S9 from Phase Ib Pharmacodynamic Study of the MNK Inhibitor Tomivosertib (eFT508) Combined With Paclitaxel in Patients With Refractory Metastatic Breast Cancer
Cristiano Ferrario and John Mackey and Karen A. Gelmon and Nathalie Levasseur and Poul H. Sorensen and Htoo Zarni Oo and Gian L. Negri and Veronica W.L. Tse and Sandra E. Spencer and Grace Cheng and Gregg B. Morin and Sonia del Rincon and Tiziana Cotechini and Christophe Gonçalves and Charles C.T. Hindmarch and Wilson H. Miller and Mehdi Amiri and Tayebeh Basiri and Victor Villareal-Corpuz and Sam Sperry and Kevin Gregorczyk and Gonzalo Spera and Nahum Sonenberg and Michael Pollak
DOI: 10.1158/1078-0432.28332724
02/2025Supplementary Table S7 from Phase Ib Pharmacodynamic Study of the MNK Inhibitor Tomivosertib (eFT508) Combined With Paclitaxel in Patients With Refractory Metastatic Breast Cancer
Cristiano Ferrario and John Mackey and Karen A. Gelmon and Nathalie Levasseur and Poul H. Sorensen and Htoo Zarni Oo and Gian L. Negri and Veronica W.L. Tse and Sandra E. Spencer and Grace Cheng and Gregg B. Morin and Sonia del Rincon and Tiziana Cotechini and Christophe Gonçalves and Charles C.T. Hindmarch and Wilson H. Miller and Mehdi Amiri and Tayebeh Basiri and Victor Villareal-Corpuz and Sam Sperry and Kevin Gregorczyk and Gonzalo Spera and Nahum Sonenberg and Michael Pollak
DOI: 10.1158/1078-0432.28332700
02/2025Data from Phase Ib Pharmacodynamic Study of the MNK Inhibitor Tomivosertib (eFT508) Combined With Paclitaxel in Patients With Refractory Metastatic Breast Cancer
Cristiano Ferrario and John Mackey and Karen A. Gelmon and Nathalie Levasseur and Poul H. Sorensen and Htoo Zarni Oo and Gian L. Negri and Veronica W.L. Tse and Sandra E. Spencer and Grace Cheng and Gregg B. Morin and Sonia del Rincon and Tiziana Cotechini and Christophe Gonçalves and Charles C.T. Hindmarch and Wilson H. Miller and Mehdi Amiri and Tayebeh Basiri and Victor Villareal-Corpuz and Sam Sperry and Kevin Gregorczyk and Gonzalo Spera and Nahum Sonenberg and Michael Pollak
DOI: 10.1158/1078-0432.c.7654993
02/2025Phase Ib Pharmacodynamic Study of the MNK Inhibitor Tomivosertib (eFT508) Combined With Paclitaxel in Patients With Refractory Metastatic Breast Cancer
Clinical Cancer Research
Cristiano Ferrario and John Mackey and Karen A. Gelmon and Nathalie Levasseur and Poul H. Sorensen and Htoo Zarni Oo and Gian L. Negri and Veronica W.L. Tse and Sandra E. Spencer and Grace Cheng and Gregg B. Morin and Sonia del Rincon and Tiziana Cotechini and Christophe Gonçalves and Charles C.T. Hindmarch and Wilson H. Miller, Jr and Mehdi Amiri and Tayebeh Basiri and Victor Villareal-Corpuz and Sam Sperry and Kevin Gregorczyk and Gonzalo Spera and Nahum Sonenberg and Michael Pollak
DOI: 10.1158/1078-0432.CCR-24-0841
02/2025Supplementary Fig. S6 from Phase Ib Pharmacodynamic Study of the MNK Inhibitor Tomivosertib (eFT508) Combined With Paclitaxel in Patients With Refractory Metastatic Breast Cancer
Cristiano Ferrario and John Mackey and Karen A. Gelmon and Nathalie Levasseur and Poul H. Sorensen and Htoo Zarni Oo and Gian L. Negri and Veronica W.L. Tse and Sandra E. Spencer and Grace Cheng and Gregg B. Morin and Sonia del Rincon and Tiziana Cotechini and Christophe Gonçalves and Charles C.T. Hindmarch and Wilson H. Miller and Mehdi Amiri and Tayebeh Basiri and Victor Villareal-Corpuz and Sam Sperry and Kevin Gregorczyk and Gonzalo Spera and Nahum Sonenberg and Michael Pollak
DOI: 10.1158/1078-0432.28332733
02/2025Supplementary Fig. S8 from Phase Ib Pharmacodynamic Study of the MNK Inhibitor Tomivosertib (eFT508) Combined With Paclitaxel in Patients With Refractory Metastatic Breast Cancer
Cristiano Ferrario and John Mackey and Karen A. Gelmon and Nathalie Levasseur and Poul H. Sorensen and Htoo Zarni Oo and Gian L. Negri and Veronica W.L. Tse and Sandra E. Spencer and Grace Cheng and Gregg B. Morin and Sonia del Rincon and Tiziana Cotechini and Christophe Gonçalves and Charles C.T. Hindmarch and Wilson H. Miller and Mehdi Amiri and Tayebeh Basiri and Victor Villareal-Corpuz and Sam Sperry and Kevin Gregorczyk and Gonzalo Spera and Nahum Sonenberg and Michael Pollak
DOI: 10.1158/1078-0432.28332727
02/2025Supplementary Table S3 from Phase Ib Pharmacodynamic Study of the MNK Inhibitor Tomivosertib (eFT508) Combined With Paclitaxel in Patients With Refractory Metastatic Breast Cancer
Cristiano Ferrario and John Mackey and Karen A. Gelmon and Nathalie Levasseur and Poul H. Sorensen and Htoo Zarni Oo and Gian L. Negri and Veronica W.L. Tse and Sandra E. Spencer and Grace Cheng and Gregg B. Morin and Sonia del Rincon and Tiziana Cotechini and Christophe Gonçalves and Charles C.T. Hindmarch and Wilson H. Miller and Mehdi Amiri and Tayebeh Basiri and Victor Villareal-Corpuz and Sam Sperry and Kevin Gregorczyk and Gonzalo Spera and Nahum Sonenberg and Michael Pollak
DOI: 10.1158/1078-0432.28332715
02/2025Supplementary Table S4 from Phase Ib Pharmacodynamic Study of the MNK Inhibitor Tomivosertib (eFT508) Combined With Paclitaxel in Patients With Refractory Metastatic Breast Cancer
Cristiano Ferrario and John Mackey and Karen A. Gelmon and Nathalie Levasseur and Poul H. Sorensen and Htoo Zarni Oo and Gian L. Negri and Veronica W.L. Tse and Sandra E. Spencer and Grace Cheng and Gregg B. Morin and Sonia del Rincon and Tiziana Cotechini and Christophe Gonçalves and Charles C.T. Hindmarch and Wilson H. Miller and Mehdi Amiri and Tayebeh Basiri and Victor Villareal-Corpuz and Sam Sperry and Kevin Gregorczyk and Gonzalo Spera and Nahum Sonenberg and Michael Pollak
DOI: 10.1158/1078-0432.28332712
02/2025Eukaryotic Elongation Factor 2 Kinase EFK-1/eEF2K promotes starvation resistance by preventing oxidative damage inC. elegans
Junran Yan and Forum Bhanshali and Chiaki Shuzenji and Tsultrim T. Mendenhall and Xuanjin Cheng and Pamela Bai and Gahan Diwan and Donna Seraj and Joel N. Meyer and Poul H. Sorensen and Jessica H. Hartman and Stefan Taubert
DOI: 10.1101/2024.03.20.585993
03/2024Pediatric Brain Tumours: Lessons from the Immune Microenvironment
Current Oncology
Betty Yao and Alberto Delaidelli and Hannes Vogel and Poul Sorensen
DOI: 10.3390/curroncol30050379
05/2023RNA modifications in brain tumorigenesis
Acta Neuropathologica Communications
Albert Z. Huang and Alberto Delaidelli and Poul H. Sorensen
DOI: 10.1186/s40478-020-00941-6
12/2020 - Research
-
Role of transforming growth factor beta (TGFß) in pediatric neoplasia
One of the hallmarks of advanced malignancies is the production by tumour cells of TGFß and, paradoxically, the inhibition of TGFß signaling in tumour cells. The group has found through microarray studies that TGFß is markedly up-regulated by the EN protein, and that EN inhibits TGFß signaling. Therefore EN transformation appears to an excellent model of the role TGFß in neoplasia.Role of the insulin-like growth factor 1 receptor (IGFRI) pathway in pediatric neoplasia
IGFs are expressed in many pediatric malignancies, and it is thought that the IGFRI axis plays a key role in oncogenesis in these tumours. The group recently found that the ETV6-NTRK3 (EN) fusion protein fails to transform IGFRI -/- murine fibroblasts, but that re-introduction of IGFRI restores EN transformation activity. Ongoing studies are assessing mechanisms by which IGFRI complements dominantly acting fusion proteins.Cyclin D1 regulation in pediatric solid tumours
The abundance of cyclin D1 is rate-limiting in G1 phase progression. The group has found, by microarray and other studies, that several pediatric sarcoma translocation-associated fusion oncoproteins markedly up-regulate cyclin D1 expression. The mechanism of over-expression is currently being assessed by studying transcriptional regulation (such as by the AP-1 and NFKB transcription factors), regulation by the generation of reactive oxygen species (ROS), and regulation of cyclin D1 protein stability by the PI3-kinase-AKT pathway.Honours & AwardsUBC Pathology Award for Excellence in Research and Discovery - 2001
UBC Pathology Award for Excellence in Research and Discovery - 2014
Targeting MondoA: A potential new treatment for childhood leukemia
Dr. Poul Sorensen is co-senior author of a study that could lead to a potential new treatment for one of the most common forms of childhood leukemia.