Publications
Real-world peanut OIT in infants may be safer than non-infant preschool OIT and equally effective
Soller, L., Carr, S., Kapur, S. Rex, G.R., McHenry, M. Cook, V.E., Leo, S., Wong, T., Vander Leek, T.K., Gerstner, T.V., Yeung, J., Abrams, E.M., Mak, R., Hildebrand, K.J., Erdle, S.C., Cameron, S.B., Chan, E.S. -world peanut OIT in infants may be safer than non-infant preschool OIT and equally effective. (2022). Journal of Allergy and Clinical Immunology: In Practice, 10(4), 1113-1116.e1. doi: 10.1016/j.jaip.2021.12.009
Real-world preschool peanut oral immunotherapy may be safer in infants than non-infant preschoolers, in which no infants have severe reactions. It is equally effective: 81% of patients tolerated 4000 mg protein (and all patients tolerated ≥1,000 mg) at the 1-year food challenge.
Development of IMPAACT (Impairment Measure for Parental Food Allergy-Associated Anxiety and Coping Tool), a validated tool to screen for food allergy-associated parental anxiety
To, S., Westwell-Roper, C., Soller, L., Stewart, S.E., Chan, E.S. (2022). Development of IMPAACT (Impairment Measure for Parental Food Allergy-Associated Anxiety and Coping Tool), a validated tool to screen for food allergy-associated parental anxiety. Annals of Allergy, Asthma, and Immunology, 129(4), 451-460e3. doi: 10.1016/j.anai.2022.02.020.
Parents commonly experience anxiety owing to their children's food allergies (FAs). Although FA-specific anxiety screening tools for adult and pediatric patients exist, a tool for parents with children with food allergy is lacking. The objective of this study was to develop and validate a tool that measures parental anxiety related to their child's FA. To construct the instrument, items were developed based on consultations with stakeholders and review of existing literature. The instrument was then pilot tested, and items were modified based on relevance, importance, item-total correlations, and fit with the instrument's overall factor structure. The modified instrument was validated through assessing internal validity (reliability), convergent and discriminant validity, concurrent validity, and practical usefulness at 2 time points (pre-coronavirus disease 2019 and current). The scale showed excellent reliability (Cronbach's α = 0.95). It had a 4-factor structure that was replicated at the 2 time points. The 4 subscales were moderately correlated (between r = 0.438 and 0.744). The scale showed excellent convergent and discriminatory validity, correlating moderately with State Trait Anxiety Inventory and Generalized Anxiety Disorder, and highly with Food Allergy Quality of Life-Parental Burden. It also showed excellent concurrent validity, differentiating among many external variables. Most importantly, it successfully differentiated parents in need of psychological support for problems related to their child's FA. The Impairment Measure for Parental Food Allergy-associated Anxiety and Coping Tool fills a gap in the existing literature as a validated screening tool for parental anxiety associated with a child's FA, employing a multi-factor structure addressing multiple dimensions of anxiety and its functional impacts. It has excellent internal and external validity and is well-suited for use in both research and clinical settings to quickly determine which parents of children with FA are in need of further psychological support.
Food-allergy-specific anxiety and distress in parents of children with food allergy: A systematic review
Westwell-Roper, C., To, S., Andjelic, G., Lu, C., Lin, B., Soller, L., Chan, E.S., Stewart, S.E. (2022). Food-allergy-specific anxiety and distress in parents of children with food allergy: A systematic review. Pediatric Allergy and Immunology, 33(1), e13695. doi: 10.1111/pai.13695.
Parenting a child with food allergy (FA) can lead to impaired quality of life and family functioning. Anxiety is a critical component of FA-associated distress and a potential target for therapeutic intervention. This systematic review aimed to clarify the concept of FA-specific anxiety (FAA) and its antecedents, consequences, and correlates and to determine the extent to which existing FA-specific outcome measures capture symptoms of parental distress and FAA. MEDLINE, EMBASE, PsycINFO, and CENTRAL were searched for qualitative and quantitative studies examining distress or anxiety in parents of children with FA through August 2020. This review was registered with PROSPERO (CRD42020208316) and conducted in accordance with PRISMA guidelines. Ninety-eight studies were included in the final narrative synthesis. Most participants were mothers, and reporting of demographic data was limited. Parents identified anxiety as the most burdensome form of FA-specific emotional distress. Several allergy-related factors as well as medical and psychosocial interventions were associated with reduced parental anxiety and distress. However, affective, cognitive, and behavioral dimensions of FAA were only partially addressed by existing measures for general anxiety symptoms and FA-specific parental factors. FAA contributes to distress and functional impairment among parents of children with FA. Current FA-specific parent measures fail to adequately capture dimensions of FAA, suggesting that further work is needed to improve the assessment and monitoring of FAA and its impacts. Characterization of this construct represents an initial step in developing standardized methods for assessing and monitoring FAA in clinical populations.
A practical focus on legume oral immunotherapy
Chua, G.T., Chan, E.S. (2022). A practical focus on legume oral immunotherapy. Journal of Food Allergy (USA), 4(2), 144-147(4). doi.org/10.2500/jfa.2022.4.220006
Legumes other than peanut are an important source of protein and consist of a wide variety of species, such as soy, peas, chickpeas, lentils, and lupin. Due to their health benefits and the rising popularity of veganism, legume consumption has increased. Legume allergy, cross-sensitization, and cross-reactivity between different species have been reported in the literature and are increasingly recognized. Unlike peanut, oral immunotherapy (OIT) for non-peanut legumes has not been well studied and published protocols are lacking. Future studies are needed to provide real-world data on the safety and effectiveness of non-peanut legume OIT, and whether desensitization to one legume leads to desensitization to other legumes in patients with multiple legume allergy. Nevertheless, due to the abundance of clinical trial and real-world data for peanut OIT, it is reasonable to use protocols that substitute peanut protein with other legume protein when desensitizing individuals with non-peanut legume allergy. Clinicians who are starting to offer legume OIT in their practices may consider starting with preschoolers, an age group for whom real-world data has shown the greatest safety and effectiveness.
The Case for Prompt Salvage Infant Peanut Oral Immunotherapy Following Failed Primary Prevention
Chua, G.T., Greenhawt, M., Shaker, M., Soller, L., Abrams, E.M., Cameron, S.B., Cook, V.E., Erdle, S.C., Fleischer, D.M., Mak, R., Vander Leek, T.K., Chan, E.S. (2022). The Case for Prompt Salvage Infant Peanut Oral Immunotherapy Following Failed Primary Prevention. Journal of Allergy and Clinical Immunology: In Practice, 10(10), 2561-2569. 10.1016/j.jaip.2022.05.040.
Recent guideline recommendations have shifted from recommending prolonged avoidance of allergenic foods in the first 3 years of life to a primary prevention approach involving the deliberate early introduction to infants at risk of developing food allergy. Despite this, some infants, especially those with severe eczema who are at highest risk for developing peanut allergy, fail to receive the preventative benefits of early peanut introduction due to hesitancy and other factors. Difficulty adhering to regular ingestion after introduction further reduces the effectiveness of primary prevention. As emerging real-world evidence has demonstrated that performing peanut oral immunotherapy (OIT) among infants is effective and safe, peanut OIT could be a treatment option for infants with peanut allergy. This review discusses the benefits, risks, and barriers to offering peanut OIT to infants who fail primary prevention strategies. We propose the novel concept that infants with peanut allergy be offered peanut OIT as soon as possible after failed peanut introduction through a shared decision-making process with the family, where there is a preference for active management rather than avoidance.
Patient selection for milk and egg ladders using a food ladder safety checklist
Chua, G.T, Chan, E.S., Yeung, J, Cameron, S.B., Soller, L, Williams, B.A., Chomyn, A., Vander Leek, T.K., Abrams, E.M., Mak, R., Wong, T. (2022). Patient selection for milk and egg ladders using a food ladder safety checklist. Allergy, Asthma & Clinical Immunology, 18(51).
A food ladder is a form of home-based dietary advancement therapy that gradually increases exposure to an allergenic food through the gradual introduction of egg or milk containing food with increasing quantity and allergenicity from extensively heated forms, such as baked goods, to less processed products. While widely considered safe, the food ladder is not risk-free and most of the egg and milk ladder studies only included preschoolers with mild egg and milk allergies, and with no or well-controlled asthma. We propose a Food Ladder Safety Checklist to assist with patient selection using “4 A's” based on available evidence for food ladders, including Age, active or poorly controlled Asthma, history of Anaphylaxis, and Adherence.
A High Proportion of Canadian Allergists Offer Oral Immunotherapy but Barriers Remain
Mack, D.P., Soller, L., Chan, E.S., Hanna, M.A., Terpstra, C., Vander Leek, T.K., Begin, P. (2021). A High Proportion of Canadian Allergists Offer Oral Immunotherapy but Barriers Remain. Journal of Allergy and Clinical Immunology: In Practice, 9(5), 1902-1908. doi: 10.1016/j.jaip.2020.12.025
Limited data on clinical implementation of oral immunotherapy (OIT) have been reported with incomplete evaluation of barriers. The objective of this study was to survey Canadian allergists on their current practice of OIT and barriers to implementation and expansion of OIT. A survey investigating current practice and logistical and clinical barriers to offering or expanding OIT was distributed to all Canadian Society of Allergy and Clinical Immunology allergists. Of 90 responding allergists, 52.2% reported offering OIT, most commonly to peanut. Food sublingual immunotherapy was offered by 7% of allergists. Having received training for OIT was associated with currently performing OIT (P = .008); 44.7% of allergists offering OIT had received training on OIT, and 81.4% not offering OIT had no training. A total of 87% of allergists performing OIT reported lack of efficacy data and lack of support staff and clinic space, and concerns about increased oral challenges (84%) were “moderately” to “extremely” important barriers to expanding OIT. For clinicians not offering OIT, concerns about safety (95%), after-hours support (95%), efficacy (93%), medicolegal risk (93%), and long-term practice implications (93%) were prioritized as significant barriers. Qualitative assessment suggested concerns about the practical challenges associated with OIT, the need for increased safety and efficacy data, and a desire for OIT guidelines and training. The implementation of OIT faces many barriers, both clinical and logistical. Increasing high-quality safety and efficacy data may support those hesitant to offer OIT, and improving funding may address the practical infrastructure challenges. In addition, training will help expand access for allergists interested in performing OIT.
First Real-World Effectiveness Analysis of Preschool Peanut Oral Immunotherapy
Soller, L., Abrams, A.M., Carr, S., Kapur, S., Rex, G.A., Leo, S., McHenry, M., Vander Leek, T.K., Yeung, J., Cook, V.E., Wong, T., Hildebrand, K.J., Mak, R., Gerstner, T.V., Cameron, S.B., Chan, E.S. (2021). First real-world effectiveness analysis of preschool peanut oral immunotherapy. Journal of Allergy and Clinical Immunology: In Practice, 9(3), 1349-1356.e1. 10.1016/j.jaip.2020.10.045
We sought to determine the effectiveness of preschool P-OIT after 1 year of maintenance. 117 preschoolers (9-70 months) with at least 1 objective reaction to peanut completed a follow-up Oral Food Challenge (OFC) to cumulative 4000 mg protein after 1 year on 300 mg peanut daily maintenance. Effectiveness of desensitization was defined as proportion of patients with a negative follow-up OFC. 92 (78.6%) patients had a negative OFC and 115 (98.3%) tolerated a cumulative dose of greater than or equal to 1000 mg. For the 25 (21.4%) who reacted, their threshold increased by 3376 mg (95% CI, 2884-3868) from baseline to follow-up; 17 (14.5%) patients experienced grade 1 reactions, 7 (6.00%) grade 2, and 1 (0.85%) grade 3. Two patients (1.71%) received epinephrine associated with P-OIT, and 1 (0.85%) went to the emergency department. Our data demonstrate that real-world preschool P-OIT is effective after 1 year of maintenance for those who received a follow-up OFC. For those who reacted, their threshold increased sufficiently to protect against accidental exposures. P-OIT should be considered for preschoolers as an alternative to current recommendations to avoid peanut.
Allergic Reactions to Emerging Food Allergens in Canadian Children
Soller, L., La Vieille, S., Cameron, S.B., Mak, R., Cook, V.E., Gerdts, J., Chan, E.S. (2021). Allergic reactions to emerging food allergens in Canadian children. Allergy, Asthma & Clinical Immunology, 17(71). doi.org/10.1186/s13223-021-00573-y
Most Canadian food allergy data has focused on Health Canada’s priority food allergens. This study describes which non-priority (emerging) food allergens were most commonly reported by Canadian parents and categorized/confirmed by allergists. A secondary aim was to describe severity of allergic reactions to emerging allergens. Parents reported allergic reactions to emerging food allergens experienced by their child (< 18 years) which occurred in the past 12 months, and allergists categorized/confirmed them according to likelihood of IgE-mediated food allergy. Of 68 eligible patients completing the survey, the most commonly reported emerging allergens were fruits/vegetables (58.8%), seeds (22.1%), legumes (19.1%) and other (11.8%). Median allergist ranking for legumes was ‘probable’ IgE-mediated food allergy, ‘possible’ for seeds and fruits/vegetables, and ‘unlikely’ for other. Median reaction severity was mild for legumes, and moderate for seeds, fruits/vegetables, and other. Our study highlights that non-priority food allergens, namely legumes and seeds, can lead to probable/likely allergic reactions in Canadian children. These food allergens are increasing in popularity in the Canadian diet, which could lead to increasing reports of allergic reactions. More research is needed to confirm reports of reactions to emerging allergens, and to document their inclusion as ingredients in packaged foods.
Home-Based Peanut Oral Immunotherapy for Low-Risk Peanut-Allergic Preschoolers during the COVID-19 Pandemic and Beyond
Chua, G.T., Chan, E.S., Soller, L.S., Cook, V.E., Vander Leek, T.K., Mak, R. (2021). Home-based peanut oral immunotherapy for low-risk peanut-allergic preschoolers during the COVID-19 pandemic and beyond. Frontiers in Allergy, (forthcoming). 10.3389/falgy.2021.725165
The COVID-19 pandemic has led to the deprioritization of non-emergency services, including oral food challenges and the initiation of oral immunotherapy (OIT) for food-allergic children. Recent studies have suggested that home-based peanut OIT could be a safe and effective option for low-risk peanut-allergic children. Between 1 September 2020 and 31 January 2021, nine preschoolers with a history of mild allergic reactions to peanut underwent home-based peanut OIT. Eight of them (88.9%) completed the build-up phase at home in 11-28 weeks, tolerating a daily maintenance dose of 320mg peanut protein. During the build-up, six patients (75.0%) reported urticaria, three (33.3%) reported gastrointestinal tract symptoms, and one (14.3%) reported oral pruritis. None developed anaphylaxis, required epinephrine, or attended emergency services related to OIT. One or two virtual follow-up visits were completed per patient during the build-up phase. Our preliminary work shows that home-based OIT could be offered to low-risk preschoolers during the COVID-19 pandemic when non-emergency services are limited and could be considered beyond the pandemic, especially for families living in rural or remote areas who may otherwise be unable to access OIT.
Canadian Food Ladders for Dietary Advancement in Children with IgE-Mediated Allergy to Milk and/or Egg
Chomyn, A., Chan, E.S., Yeung, J., Vander Leek, T.K., Williams, B.A., Soller, L., Abrams, E.M., Mak, R., Wong, T. (2021) Canadian food ladders for dietary advancement in children with IgE-mediated allergy to milk and/or egg. Allergy, Asthma & Clinical Immunology, 17(83). doi.org/10.1186/s13223-021-00583-w
Food ladders are clinical tools already widely used in Europe for food reintroduction in milk- and egg-allergic children. Previously developed milk and egg ladders have limited applicability to Canadian children due to dietary differences and product availability. Herein we propose a Canadian version of cow’s milk and egg food ladders and discuss the potential role that food ladders may have in the care of children with IgE-mediated allergies to cow’s milk and/or egg, as either a method of accelerating the acquisition of tolerance in those who would outgrow on their own, or as a form of modified oral immunotherapy in those with otherwise persistent allergy.
Caregiver Views on Virtual Management of Food Allergy: A Mixed Methods Study
Chan, E.S., Jeimy, S., Hannah, M., Cook, V.E., Mack, D.P., Abrams, E.M., Soller, L., Wong, T., Protudjer, J.L. (2021). Canadian views on virtual management of food allergy: A mixed methods study. Pediatric Allergy and Immunology, 1-4. doi.org/10.1111/pai.13539
During the first months of the COVID‐19 pandemic, healthcare acutely switched almost exclusively to virtual delivery, with little precedent, and even less knowledge about caregivers’ comfort with such delivery. It is unlikely that we will ever return to exclusively in‐person care (3, 4). Thus, there is a critical, but currently unstudied, need to understand caregivers’ attitudes toward virtual food allergy management. To address this knowledge gap, we performed a mixed‐methods study in which we sought to examine caregivers’ views on virtual food allergy care.
Grass pollen Allergy as an Anaphylaxis Co-Factor during Peanut Oral Immunotherapy
Chua, G.T., Chan, E.S., Soller, L., Cameron, S.B. (2021). Grass pollen allergy as an anaphylaxis co-factor during peanut oral immunotherapy. Annals of Allergy, Asthma & Immunology, 127(2), 263-264. doi.org/10.1016/j.anai.2021.04.037
Grass pollen allergy, typically associated with non–life-threatening symptoms, such as rhinoconjunctivitis, is one of the most common allergies worldwide. Rarely, anaphylaxis occurs after grass pollen exposure in children. Oral immunotherapy (OIT) for the treatment of food allergy has been gaining popularity in the last decade as evidence on methodology, effectiveness, and safety has progressed. Studies have revealed that patients with peanut allergy on Oral Immunotherapy (OIT) with seasonal allergic rhinitis experience dose-related adverse events more frequently with seasonal patterns. Nevertheless, pollen allergy as an anaphylaxis cofactor while on peanut OIT (POIT) has never been described.
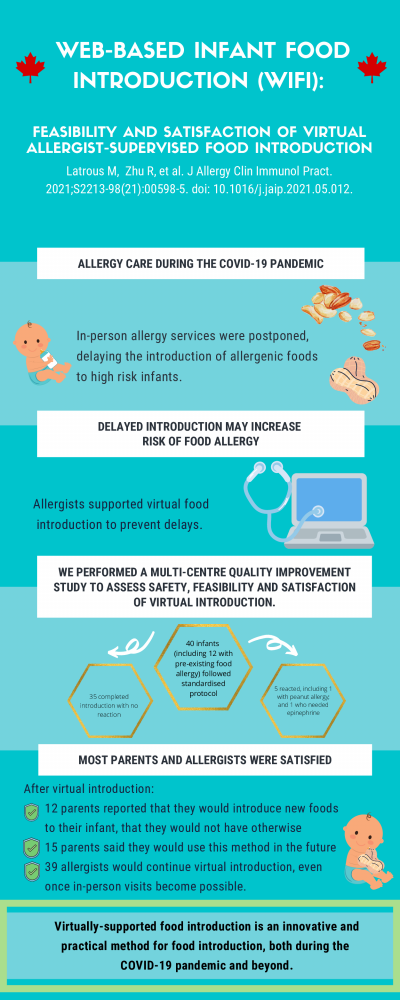
Web-based Infant Food Introduction (WIFI): Feasibility and satisfaction of virtual allergist-supervised food introduction
Latrous, M., Zhu, R., Mack, S.P., Soller, L., Chan, E.S., Jeimy, S., Hannah, M., Abrams, E.M., Cameron, S.B., Cook, V.E., Erdle, S., Protudjer, J.L., Wong, T. (2021). Web-based infant food introduction (WIFI): feasibility and satisfaction of cirtual allergist-supervised food introduction. The Journal of Allergy and Clinical Immunology: In Practice, 9(9), 3521 – 23.e1. 10.1016/j.jaip.2021.05.012
The coronavirus disease 2019 (COVID-19) pandemic has resulted in the modification and postponement of many allergy clinic services. As a result, effective implementation of food allergy prevention poses a challenge. We performed a Canada-wide multicentre quality improvement initiative to assess the safety and feasibility of virtually supported introduction of any allergenic food in high-risk infants. In addition, we explored both allergist and caregiver satisfaction of the virtually supported food introduction. Between May 14 and October 31, 2020, 40 infants (median age (months): 9, IQR 7-11) underwent virtually supported food introduction. Both the median caregiver and allergist satisfaction scores of virtually supported food introduction were 99 of 100. 83% of caregivers stated they would opt into this method in the future if it meant shorter wait times, and 98% of allergists sated they would continue to offer virtually supported food introduction even when in-person visits become fully available.
How to Incorporate Oral Immunotherapy into Your Clinical Practice
Abrams, E.M., Erdle, S.C., Cameron, S.B., Soller, L., Chan, E.S. (2021). How to incorporate oral immunotherapy into your clinical practice. Current Allergy and Asthma Reports, 21(30). doi.org/10.1007/s11882-021-01009-8
This review discussed how to best incorporate oral immunotherapy (OIT) into your clinical practice based on recent evidence and guidelines, and addressed controversies. Recent data from both randomized clinical trials and real-world studies show OIT is especially safe and effective in preschoolers, while avoidance may be less safe than previously thought. OIT guidelines support its use outside of research. OIT can be safely and effectively incorporated into your clinical practice, with careful planning and consideration of scenarios where benefits outweigh risks. Baseline oral food challenges are necessary in clinical trials, but in clinical practice, these are best done when the history is unclear due to resource limitations. There is a role for both regular food and FDA-approved products. Future research should focus on optimizing safety and adherence in the real-world setting.
The Clinically Important Impact of Preschool Food Oral Immunotherapy on Parental Quality of Life
Chooniedass, R., Soller, L., Kapur, S., Rex, G., McHenry, M., Vander Leek, T., Mak, R., Gerstner, T., Carr, S., Chan, E.S. (2021). The clinically important impact of preschool food oral immunotherapy on parental quality of life. The Journal of Allergy and Clinical Immunology, 147(2). 10.1016/j.jaci.2020.12.457
Our group previously showed the safety of peanut oral immunotherapy (OIT) in 270 preschool-aged children (0.4% had severe reactions). We sought to determine the clinical impact of preschool OIT on parental quality of life. Between May 2019 – August 2020, 29 patients aged 9-32.2 months completed OIT and the Food Allergy Quality of Life– Parental Burden (FAQL-PB) questionnaire at baseline and end of build-up. There was a significant improvement in FAQL-PB from baseline to end of build-up with a median change in score of -14 (IQR: -33.5, -7.5; p<0.00001). Our real-world study found that preschool OIT improves parent QoL from baseline to end of build-up.
Virtually Supported Home Peanut Introduction During COVID-19 for At-Risk Infants
Mack, D.P., Hanna, M.A., Abrams, E.M., Wong, T., Soller, L., Erdle, S.C., Jeimy, S., Protudjer, J.L. (2020). Virtually Supported Home Peanut Introduction During COVID-19 for At-Risk Infants. The Journal of Allergy and Clinical Immunology: In Practice, 8(8), 2780-2783. doi.org/10.1016/j.jaip.2020.05.048
Infants at risk for development of peanut allergy have very limited access to allergist assessment for the prevention of peanut allergy during the COVID-19pandemic. This study shows that virtually supported infant home introduction represents a practical and safe alternative to avoid delays in peanut introduction. We report the first known use of a virtually supported home introduction option for infants at risk of developing peanut allergy.
Parents of Children with Food Allergy: A Qualitative Study Describing Needs and Identifying Solutions
Chooniedass, R., Soller, L., Hsu, E., To, S., Cameron, S.B., Chan, E.S. (2020). Parents of Children with Food Allergy: A Qualitative Study Describing Needs and Identifying Solutions. Annals of Allergy, Asthma & Immunology, 125(6), 674-679. doi.org/10.1016/j.anai.2020.05.014
We sought to describe parental experiences when caring for a child with food allergy and to review the resources parents need to manage living with a child with food allergy and more specifically how they would want these resources delivered. We conducted 7 semi-structured focus groups with 40 parents in British Columbia, Canada. Main themes that emerged were: (1) anxiety (an emotional roller coaster); (2) a transformational journey (the waiting game, loss of normalcy, strained relationships and mistrust, and financial challenges); and (3) the need for resources (day to day management, ages and stages, mental health supports, and “the dream”). In conclusion, an in-person allied health care team is needed to provide an integrated, patient-centered approach for how families can live and manage food allergies. Credible information and resources should be provided.
Current Tools Measuring Anxiety in Parents of Food-Allergic Children are Inadequate
Soller, L., To, S., Hsu, E., Chan, E.S. (2020). Current Tools Measuring Anxiety in Parents of Food-Allergic Children are Inadequate. Pediatric Allergy and Immunology, 31, 678-685. 10.1111/pai.13260
In the context of food allergy, excessive parental anxiety can be maladaptive and lead to unnecessary restriction of social activities. No validated tool exists to measure food allergy-associated anxiety (FAAA). This study sough to explore factors associated with parental FAAA, determine sensitivity and specificity of using generic state anxiety measure – State-Trait Anxiety Inventory, and determine whether validated tools for generalized anxiety for food allergy – specifically quality of life (QoL) could be used as surrogates for FAAA. Canadian parents of food-allergic children completed an online survey. Without a validated tool for FAAA, a visual analogue scale was used to assess parent-reported FAAA. Factors positively associated with FAAA included parental burden, risk perception, state anxiety, intolerance of uncertainty and perceived severity of child's food allergy; personal/family history of mental health was negatively associated. Sensitivity and specificity of state anxiety were 68.6% and 70.0%. Factor analysis revealed that state anxiety and QOL were correlated (r = 0.54, P < .001) but distinct constructs. Our study identified factors associated with FAAA, and determined that generic anxiety and QOL tools do not accurately categorize parents with self-reported high FAAA.
Oral Food Challenge Implementation: The First Mixed-Methods Study Exploring Barriers and Solutions
Hsu, E., Soller, L., Abrams, E.M., Protudjer, J.L.P., Mill, C., Chan, E.S. (2020). Oral Food Challenge Implementation: The First Mixed-Methods Study Exploring Barriers and Solutions. The Journal of Allergy and Clinical Immunology: In Practice, 8(1), 149-156. doi.org/10.1016/j.jaip.2019.06.034
Because of inaccuracies in commonly used tests for food allergy, oral food challenges (OFCs) are considered the criterion standard, but OFC implementation is suboptimal. The purpose of this study was to use a mixed-methods approach to describe OFC barriers at multiple levels and investigate solutions. Surveys of Canadian allergists, pediatricians, and parents investigated barriers to offering or participating in OFCs, and possible solutions. Of 62 responding allergists, 80.6% reported performing OFCs, 72.6% reported lack of resources as an influential barrier, and 72.6% reported that creation of standard guidelines for hospital versus community OFCs would influence them to perform more OFCs. Of 101 responding pediatricians, 51.5% reported having moderate-to-extensive OFC knowledge; these pediatricians were more likely to refer to allergists who performed them (odds ratio, 2.37; 95% CI, 1.06-5.30). Of 27 pediatricians who stated they refer more to allergists who do not perform OFCs, 40.7% reported long wait times as a deterrent. The most common parent barriers from surveys (N = 110) and focus groups (N = 27) were fear and anxiety about the procedure and about experiencing reactions during OFCs, suggesting the need for better information and psychosocial resources. Efforts targeting OFC training for allergists, education for pediatricians, and standardized guidelines created with clinician and parent input (including consistent OFC information for families and guidance on which OFCs should be performed in-hospital versus the community) are likely to increase OFC acceptance.
Comparing Quality of Life in Canadian Children with Peanut, Sesame, and Seafood Allergy
Soller, L., Clarke, A.E., Lyttle, A., Chin, R., Ben-Shoshan, M., Cheuk, S., Asai, Y., Chan, E.S. (2020). Comparing Quality of Life in Canadian Children with Peanut, Sesame, and Seafood Allergy. The Journal of Allergy and Clinical Immunology: In Practice, 8(1), 352-354. doi.org/10.1016/j.jaip.2019.07.006
Children with food allergy (FA) have a worse quality of life (QOL) than children with other chronic diseases, and the impact of FA on QOL depends on various factors, including type of FA. We hypothesized that those with peanut allergy would have a worse QOL than those with seafood allergy, and those with sesame allergy would have a worse QOL than those with seafood allergy, because peanut and sesame are more difficult to avoid. Parents of children aged <13 years with peanut, sesame, or seafood allergy and enrolled in the Canadian Food Allergy Registry completed the short version of the Food Allergy Quality of life Questionnaire—Parent Form (FAQLQ-PF10) on behalf of their allergic child between March 2014 and August 2017. Older children, females, those with an increasing number of FAs, and those with a history of anaphylaxis have a worse QOL, whereas those with university-educated parents have a better QOL. Although the QOL was worse for those with peanut versus seafood allergy in the pairwise comparison, which was what we hypothesized, this difference did not persist in multivariable analyses, potentially due to the small sample for seafood allergy. We also did not observe a QOL difference between sesame and seafood allergy, again possibly due to the sample size.
Factors Associated with Increased Food Allergy-Associated Anxiety in Parents of Food-Allergic Children
Soller, L., Hsu, E., To, S., Newlove, T., Chan, E.S. (2019). Factors Associated with Increased Food Allergy-Associated Anxiety in Parents of Food-Allergic Children. Journal of Allergy and Clinical Immunology, 143(2), AB85. doi.org/10.1016/j.jaci.2018.12.262
Parents of children with food allergy can experience anxiety and difficulty coping, which can affect the child’s ability to cope with management. Predictors of parental anxiety are currently unknown. The goal of this study was to estimate the magnitude of food allergy-associated anxiety (FAAA) in Canadian parents and determine factors associated with high anxiety. Food Allergy Canada emailed parent members in Fall 2017 inviting them to complete an online survey about their FAAA using a visual analogue scale, which measured FAAA on a scale of 0 (not anxious) to 100 (very anxious). Multiple linear regression was performed to determine demographic, clinical, and psychosocial factors associated with FAAA, and standardized coefficients were calculated. Of 1,244 parents who clicked on the survey, 548 completed it (44.1%). We identified psychosocial factors associated with parental anxiety, including risk perception, intolerance of uncertainty, and parental burden. Parental burden appeared to be most strongly associated with FAAA. This will help us develop a validated diagnostic tool, so that clinicians can more easily diagnose FAAA and provide support/resources to those who need it.
First Real-World Safety Analysis of Preschool Peanut Oral Immunotherapy
Soller, L., Abrams, E.M., Carr, S., Kapur, S., Rex, G.A., Leo, S., Lidman, P.G., Yeung, J., Vander Leek, T.K., McHenry, M., Wong, T., Cook, V.E., Hildebrand, K.J., Gerstner, T.V., Mak, R., Lee, N.J., Cameron, S.B., Chan, E.S. (2019). First real-world safety analysis of preschool peanut oral immunotherapy. Journal of Allergy and Clinical Immunology: In Practice, 7(8), 2759-2767.e5. 10.1016/j.jaip.2019.04.010
In 2017, a clinical trial of 37 subjects demonstrated that preschool peanut oral immunotherapy (P-OIT) was safe, with predominantly mild symptoms reported and only 1 moderate reaction requiring epinephrine. As part of a Canada-wide quality improvement project, we sought to examine whether these findings would be applicable in a real-world setting. We administered P-OIT to 270 patients from 2017 to 2018, 243 of which reached maintenance and 27 of which dropped out (10.0%). Overall, P-OIT appeared to be safe for most patients because symptoms were generally mild and very few reactions received epinephrine (67.8% of patients experienced reactions during buildup: 36.3% grade 1, 31.1% grade 2, and 0.40% grade 4 and eleven patients (4.10%) received epinephrine), however, life-threatening reactions in a minority of patients (0.4%) can still occur. We are the first group to describe preschool P-OIT in a real-world multicenter setting.
Infographics