Who we are
Since its inception in 2011, SEARCH & PREVENT continue to serve the needs of DSEN for active surveillance of adverse drug reactions (ADRs) and effectiveness concerns of importance to policy makers with pharmacogenomics and other methods to determine the biological basis of drug harm.
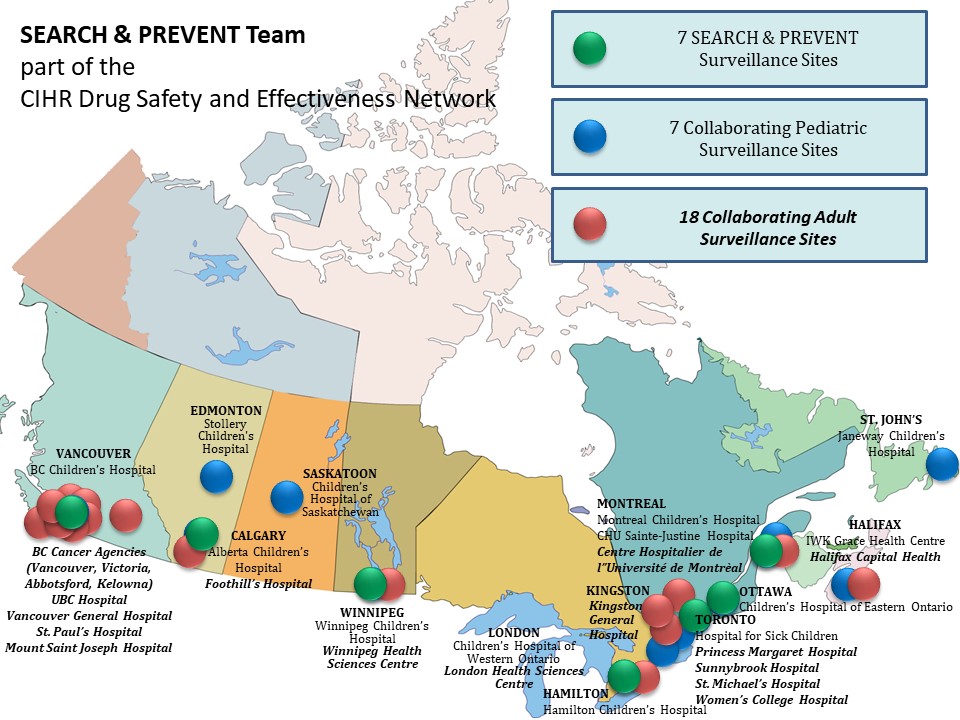
SEARCH & PREVENT is a team that builds on broad experience in active surveillance methods, pharmacogenomics expertise and established network of adverse drug reaction (ADR) surveillance clinicians in Canadian health centres. We have dedicated SEARCH & PREVENT personnel at 6 pediatric academic health centres and we have extensively collaborated with an additional 8 pediatric and 18 adult academic health centers. The role of these surveillance sites is to identify and report ADRs, and collect DNA and the detailed clinical information that is needed to evaluate potential drug risks based on patient’s genome.
The multidisciplinary nature of the team has led to clinically prioritized questions and achievable research projects, including published literature. This work has been achieved through successful collaboration among clinicians, researchers, regulators, and policymakers. The clinical recommendations resulting from the work of SEARCH & PREVENT aid incorporation of pharmacogenetic biomarkers into clinical practice which results in conducting pharmacogenetic testing. An example of such testing development and implementation into clinical practice is a pharmacogenetic test used to predict the risk for anthracycline‐induced cardiotoxicity in children.
SEARCH & PREVENT works closely with other DSEN collaborative research network groups, such as CNODES, CAN-AIM, and MAGIC, focusing on research innovation and knowledge translation and dissemination.
Methods used
SEARCH uses active surveillance to identify patients with adverse drug reactiosn (ADRs). Clinical surveillors at each academic health centre enroll identified patients with ADRs as well as patients who received the same medications without having experienced an ADR (drug‐matched controls) and subsequently collect their clinical data and biological samples for genomic analyses. All ADRs are characterized by established criteria such as Common Terminology Criteria for Adverse Events for each research project. This process provides a means to evaluate drug safety, particularly in populations often excluded from clinical trials, such as children and pregnant women.
PREVENT uses a pharmacogenomics approach to identify genetic contributors to serious ADRs. Genomic DNA is extracted from biological samples (saliva or blood). Computational biological analyses are conducted to find genetic variants that significantly impact the risk for ADRs. If these genetic variants are strongly linked to the development of an ADR, and once these findings are replicated and validated, they can be used as an ADR-predictive test to guide treatment decisions.
Our objectives
The goal of SEARCH & PREVENT team is to:
- Conduct active surveillance on drug outcomes for safety and effectiveness of DSEN policy makers priorities;
- Characterize severe adverse drug reactions (ADRs);
- Discover predictive pharmacogenomic biomarkers for the risk of drug-induced harm or therapeutic failure;
- Predict drug effectiveness and the risk of serious ADRs before therapy begins, helping to reduce the occurrence of severe ADRs through clinical and pharmacogenomics data-driven precision health strategies;
- Develop ADR predictive pharmacogenomic tools to enhance drug safety and effectiveness.
Our governance
SEARCH & PREVENT is funded by a team grant that falls under Canadian Institutes of Health Research (CIHR) Team Grant of Drug Safety and Effectiveness Network (DSEN). For details on the centralized governance please visit the CIHR website.