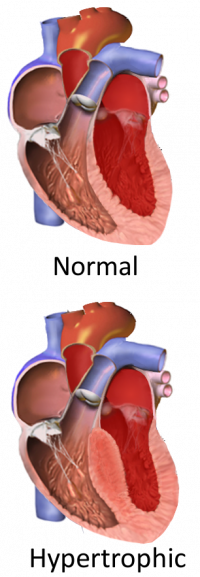
Imagine a heart, barely the size of a small fist, tirelessly filling and emptying 60 to 100 times a minute to pump blood throughout a child’s body. Now, imagine a section of that heart’s muscle — the space between the left and right ventricles — is thicker than it should be, making the heart beat too hard and impeding effective blood flow. This condition, known as hypertrophic cardiomyopathy (HCM), is one of the most common heart-muscle-related genetic diseases in children and the leading known cause of sudden cardiac arrest in youth and elite athletes.
Even with these concerning stats, HCM is relatively rare, and its symptoms range from virtually unnoticeable to severely debilitating. In fact, several individuals with the condition are not diagnosed with HCM until a family member is diagnosed or during screenings for other conditions.
When HCM symptoms are present, they can include heart palpitations, fatigue, chest pain, light-headedness, shortness of breath, and fainting episodes. Some HCM patients experience a near-total blockage of blood flow through the heart, necessitating surgery or, in rare cases, a heart transplant. The most common HCM treatments for kids include calcium channel blockers and beta-blockers, which provide some symptom relief, but do not target the underlying genetic cause.
While the majority of children with HCM have a good quality of life and average life expectancy, the risk of complications from HCM can increase with intense physical activity. These individuals, as well as those who develop more severe HCM symptoms as they age, would benefit greatly from treatments tailored to their unique genomic and physiological attributes.

But before personalized treatments for pediatric HCM are possible, we need to better understand how the disease progresses and the genetic factors that cause it. While researchers know that HCM is inherited and linked to various genetic variants, identifying the specific genetic variant impacting a child doesn’t provide enough information to develop a personalized treatment plan.
Dr. Glen Tibbits, co-director of the Cellular and Regenerative Medicine Centre at BC Children’s Hospital Research Institute and Distinguished Professor in the Department of Molecular Biology and Biochemistry and in the Department of Biomedical Physiology and Kinesiology at Simon Fraser University, says that human-induced pluripotent stem cells may be the missing link.

“Until very recently, HCM research primarily used mouse models or cardiac cell samples, but there are significant limitations associated with both of these methods,” says Dr. Tibbits.
For example, the proteins implicated in the mouse version of HCM are quite different compared to those in human HCM, and the mouse heart rate is almost 10-fold higher than in humans. Additionally, cardiac cell samples from patients do not allow for flexible disease modelling or the development of genetic controls.
“The use of human-induced pluripotent stem cells in research has really improved all of that,” continues Dr. Tibbits. “The technology is advancing our knowledge in the field of pediatric HCM in ways we never thought possible.”
Human-induced pluripotent stem cells, or hiPSC as they’re commonly abbreviated, are derived from somatic or regular body cells, as opposed to embryonic stem cells that are derived from embryos.
Dr. Tibbits, along with Dr. Shubhayan Sanatani and a team of researchers, recently published a paper in the Canadian Journal of Cardiology outlining the advances in HCM disease modelling using heart cells derived from human-induced pluripotent stem cells.
The researchers describe how hiPSC can be derived from standard blood samples from patients with HCM and then used to develop beating heart cells, or cardiomyocytes. These cardiomyocytes are genetically identical to the patient’s own cells and can be manipulated at the molecular level for a variety of research procedures.
“This is a real game changer because two patients can have the exact same HCM-related genetic variant, yet have very different symptoms and symptom severity,” says Dr. Tibbits.
Development of HCM medication is currently based on the average population and what works for most people, yet each patient has their own unique responses to these medications. Sadly, some experience serious adverse reactions or the medications provide little to no symptom relief.
“We can now test how patients will respond to different interventions and medications using hiPSC-derived cardiomyocytes before we even introduce them into a patient’s treatment protocol,” says Dr. Tibbits. “This can ultimately result in much safer and effective disease management for these kids.”
Of course, pediatric HCM researchers would like to see the ideal scenario — a complete reversal of symptoms and lifelong reduction in heart muscle thickening. Thanks to hiPSC-derived beating heart cells, this may soon be a reality.
Read more in “Advances in Hypertrophic Cardiomyopathy Disease Modelling Using hiPSC-Derived Cardiomyocytes,” Canadian Journal of Cardiology.




