Precision Diagnosis for Indigenous Families with Genetic Conditions ('Precision Diagnosis Study')
Overview
For an overview of the study, please visit the Overview page.
Health Professional Q&A
Click on the questions below for the answer to each question.
- Q: What is the purpose of this study?
-
A: Our aim is to increase access to genetic technologies and diagnosis for Indigenous (First Nations, Métis and Inuit) families by offering genome-wide sequencing to at least 200 Indigenous individuals across Canada with suspected monogenic, childhood-onset disorders.
- Q: What genetic testing will participants be offered through this study?
-
A: Participants will be offered ‘whole genome sequencing’. Whole genome sequencing differs from other genetic tests as it can examine all genetic material (coding and non-coding) in an individual to the level of the base pair, providing a detailed view of the genome. It is more comprehensive than ‘whole exome sequencing,’ which provides information about the exons of genes (the protein-coding regions). Whole genome sequencing is not routinely offered through the regular medical system at this time.
- Q: Which patients can I refer to the study?
-
A: This study is only for families who self-identify as Indigenous (First Nations, Inuit, or Métis) where there is an individual with a suspected childhood-onset, monogenic condition that has not been diagnosed through other medical tests. The proband’s condition must meet one of the following criteria:
- Multiple congenital abnormalities affecting unrelated organ systems
- Intellectual disability or global developmental delay or neurodegeneration
- The disorder is limited to a single system, is most likely due to a single gene cause, but an appropriate targeted test is not available, or available tests were non-diagnostic.
For the full, detailed eligibility criteria, please see the “Eligibility Criteria” document.
The local Principal Investigator (clinical lead) at each enrollment site will make the final decision about whether an individual is eligible for the study, after a review of the medical records. Therefore, a referral to the study does not guarantee participation. Once a patient is confirmed to be eligible, and has consented to the study, some of their unaffected family members may also be invited into the study for whole genome sequencing, to provide a genetic comparison that will help interpret the affected individual’s test results.
- Q: I think my patient might be eligible for the study – What do I do next?
-
A: Please take the following steps:
- Introduce the study to the family and provide them with the “Study Information Pamphlet” (see documents under the link for your specific province).
- If the family expresses interest, ask them to sign a “Release of Information” (ROI) form (see documents under the link for your specific province). The ROI must be signed before the study team can review your patient’s records and confirm eligibility.
- Obtain verbal consent for the study team to contact the family – alternatively, a family may wish to contact us. Contact information for your local study team will be on the “Study Information Pamphlet.”
- Some provinces may have a “Study Referral Form” that you will need to complete (see documents under your province, if applicable).
- Fax the Study Referral form and ROI to your local study team.
Below is an example of how the referral and recruitment process might go. It may vary from situation to situation. You can always contact your local study genetic counsellor (GC) if you have any questions.
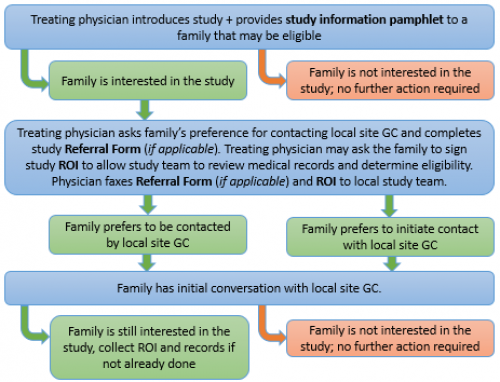
- Q: What happens after I refer a family and they are accepted into the study?
-
A: Once the study team has determined if the family you referred is eligible for the study, the study team will communicate the decision to you and to the family. For eligible families, a research appointment will be booked either by phone, video or in person. The purpose of this first appointment is to review the study in detail, obtain additional relevant medical and family history, provide pre-test genetic counselling, and if the family would like to proceed, obtain informed written consent. The next step is to organize sample collection and transport of their de-identified DNA to the BC Genome Sciences Centre in Vancouver BC. Our study team will analyze the results and any possibly disease-causing variants will be verified in a clinical lab before being reported back to you.
- Q: What happens if the study reveals a potential cause for my patient's condition?
-
A: If a disease-causing or possibly disease-causing variant is found after whole genome sequencing, a clinical report will be issued and sent back to you. As the referring physician, it is your responsibility to return this result to the family in a timely manner. A genetic counsellor from the study team will be available to be with you when you return the result to provide genetic counselling.
- Q: Will you return incidental finding (IF) results?
-
A: IFs are unexpected disease-causing variants unrelated to the original condition being investigated. Our whole genome sequencing analysis is targeted to the proband’s phenotype, and we will not purposely search for IFs. However, we still anticipate that a medically-actionable IF will be discovered in approximately 3% of participants. Return of medically actionable IFs will follow the lead of the Canadian College of Medical Genetics 2015 Position Statement, and as such, will be handled differently in adults, children, and incompetent adults:
– Competent adults: These participants will be given the option of whether they wish to receive results of any medically actionable IFs that may be discovered in them. However, those participants who choose to learn about their potential IFs, cannot choose to learn about some, and not others.
– Child participants: Pathogenic IFs will be reported back for medically actionable disorders that may have childhood-onset symptoms (for example, Neurofibromatosis 1, or Long QT syndrome). We will not disclose the status of exclusively adult-onset IFs in children unless we strongly believe it is in the child’s or family’s best interest.
– Incompetent adults: Reporting of medically actionable IF results for these participants is not optional – any medically actionable IF(s) identified will be reported back to their legal substitute decision-maker who consented to study participation.
- Q: How long will it take to get results?
-
A: We estimate a turnaround time of approximately 6 months from the time of sample collection to reporting results. However, that time may vary and we will monitor our timelines as the study progresses.
- Q: What happens if no possible disease-causing variants are identified through the study?
-
A: If there are no variants of interest identified, the study genetic counsellor will contact the family to let them know (unless the referring physician prefers to do so). The study genetic counsellor will also write a research results letter back to the referring physician explaining that no reportable variants were found at this time, with a 'research report' attached. The whole genome sequencing results will likely be re-analyzed during the course of the study as our knowledge of genetics is rapidly evolving. New information about a participant’s result may arise over the course of the study. You will be informed of any new clinically actionable results that may arise over the course of the study.
Documents
Click on the province/territory below for the documents related to your region.
-
British Columbia/Yukon
-
- Study Information Pamphlet: This pamphlet, aimed at study participants, provides a brief overview of the study and of whole genome sequencing technology in lay language. You can provide this pamphlet to families you think may be eligible for the study. Being in “pamphlet” format, this document is meant to be printed double-sided and tri-folded.
- Release of Information Form: Before being enrolled in the study, families must sign and return this form to the Silent Genomes study team so we can confirm their eligibility. Signed forms can be sent to your local study genetic counsellor with your “Study Referral Form” (Vancouver site: Karen Jacob, fax: 604-875-2376 / Victoria site: Sarah McIntosh, Fax: 250-472-4283).
- Study Referral Form: If you would like to refer a patient to the study, please fax this form (along with a signed “Release of Information” form, if applicable), to your local study genetic counsellor (Vancouver site: Karen Jacob, Fax: 604-875-2376 / Victoria site: Sarah McIntosh, Fax: 250-472-4283).
- Alberta (Calgary)
-
- Study Information Pamphlet: This study pamphlet provides a brief summary of the study and the genetic test we offering through the study (whole genome sequencing). Being in “pamphlet” format, this document is meant to be printed double-sided and tri-folded.